What is a Polymer?
What is a Polymer?
In Michael M. Coleman and Paul C. Painter’s book, Fundamentals of Polymer Science: An Introductory Text, they described a Polymer as a large molecule, or macromolecule, composed of many repeated subunits. Polymer actually means “many-parts” (poly-, “many” + -mer, “parts”) in Greek. It can be both synthetic and natural.
Natural Polymers
Synthetic Man Made Polymers
Rubbers and plastics are examples of polymers. Polymers are very big molecules with long chains of carbon atoms.
Polymers are made from smaller units called monomers. The chemical skeletal structures may be linear, cyclic or branched. Many monomers are small unsaturated molecules produced by the cracking of crude oil fractions. These monomers have the carbon to carbon double covalent bonds.
When one monomer is polymerized, the resultant polymer is called a homopolymer. Examples include Polyethylene, Polystyrene, and Polytetrafluoroethylene (PTFE). Copolymers are derived from the polymerization of more than one type of monomer. The distribution of monomers in these copolymers can be statistical, random or alternating. Examples include ethylene-propylene and fluorocarbon elastomers (vinylidene fluoride and hexafluoropropylene). Terpolymers are three-monomer-unit polymers, such as Ethylene-Propylene-Diene (EPDM) and specialty Fluorocarbon grades.
Polymerisation is a term used for the process in which a polymer is made by the joining up of monomer units.
A History of Polymers used in The Fluid Sealing Industry
Charles Goodyear (Goodyear Tires) was the first to improve the natural elastic properties of rubber by heating it with Sulphur in 1839. This was process was to become a form of Vulcanization.
In the 1930’s the macromolecule model of rubber was fully understood and by the 1950s there were rapid developments in synthetic polymers. This was the beginning of the commercial high-performance elastomers we see today.
Polymers are used for their elasticity and are ideal for O Rings. A standard O Ring can change shape, bend and be manipulated to fit a particular application.
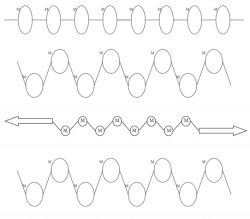
How does the O Ring retain its shape? The molecules stretch when a force is applied. When the force is removed the molecules return to their original shape. If the O Ring was stretched too much it would cause the bonds to break – losing its elasticity.
The diagrams above show the Polymer chain. The M stands for Monomer. The first two represents the Polymer chain at rest. The third is when force is applied and the chain is stretched. The fourth is the Polymer chain back in its original shape after the force has stopped.
Click here to see the full range of M Barnwell Services range of O Rings.
For more information about our Materials click here.
For more information contact a member of the team today.