Pharmaceutical Industry CIP SIP Technologies
CIP SIP Technologies = CIP (Clean-In-Place), SIP (Steam-In-Place)
CIP and SIP cleaning processes date back to the 1950s and were originally used in the food and dairy industries. Cleaning with disassembly of the equipment when changing media proved to be extremely complicated, cost-intensive, and time-consuming, so processes for cleaning in closed systems were developed.
CIP and SIP processes are also used in the pharmaceutical industry as the best method for cleaning equipment. This is because it is particularly important to avoid contamination of pharmaceutical products. Seals in the equipment must be able to withstand both the cleaning chemicals and the manufactured products.
The cleaning temperatures are determined according to the application and are usually around 90°C. The CIP process is followed by sterilisation in the SIP process. Here, active microorganisms are killed with hot water or steam at approximately 140°C, depending on the application. The use of chemical disinfectants is also possible with this process.
In the pharmaceutical industry, downtime due to cleaning and maintenance procedures should be limited or avoided altogether. But elastomer seals often fail during cleaning and sterilisation cycles.
Seals often do not meet expectations due to the:
- High cleaning temperature
- High concentration of cleaning agents (CIP)
- Long exposure time of the cleaning agents to the sealing materials
Tests of Garlock gaskets from the GYLON BIO-LINE® and GYLON BIO-LINE® PLUS series (in accordance with EHEDG Guideline No. 2 comparing the cleanability of materials) showed exceptionally good cleaning results.
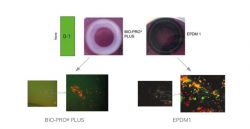
The cleanability of the seals was tested in a simulated cleaning procedure according to EHEDG guideline 2, in which the seals were examined with regard to residual microorganisms. The detection of the microorganisms was by a colour change of the nutrient medium from violet to yellow. This works in such a way that as soon as acid-forming microorganisms are present, the pH indicator bromocresol purple changes colour. The material GYLON® Style 3522 repeatedly showed exceptionally good cleaning results.
The EPDM 1 gasket did not show any yellowing during the tests, which according to EHEDG meant that the gasket was free of contamination. However, further examination using fluorescence
microscopy showed that the EPDM 1 gasket was completely contaminated. The conclusion is that the EPDM1 gasket under investigation has antimicrobial properties that caused the test procedure to fail and thus lead to a false, namely good, result. In comparison, GYLON BIO-PRO® PLUS showed no yellowing in the tests and only a few contaminants in the fluorescence microscopy.
The material GYLON® Style 3522 repeatedly showed exceptionally good cleaning results.
The exceptional cleanability of GYLON® Style 3522 gives pharmaceutical plants improved product safety, and improved process efficiency with increased product output.
M Barnwell Services also stock a variety of Garlock products including
- GYLON BIO-LOK® gaskets
- GYLON BIO-Line® Aseptic Food Grade Gaskets
- Compression Packing
- Garlock EPIX™ Seal
- PS Seal®
- PTFE Shaft Seals
- Manway Gaskets
- Rubber Expansion Joints
- KLOZURE® Bearing Isolators
- Stress Saver® Gaskets
If you would like more information about CIP SIP Technologies, contact a member of the team.
M Barnwell Services endeavour to make sure that all content is correct. Manufacturing partners assisted with the creation of this page. E & OE.